Lab Projects
Palate Development and Palatal Innervation
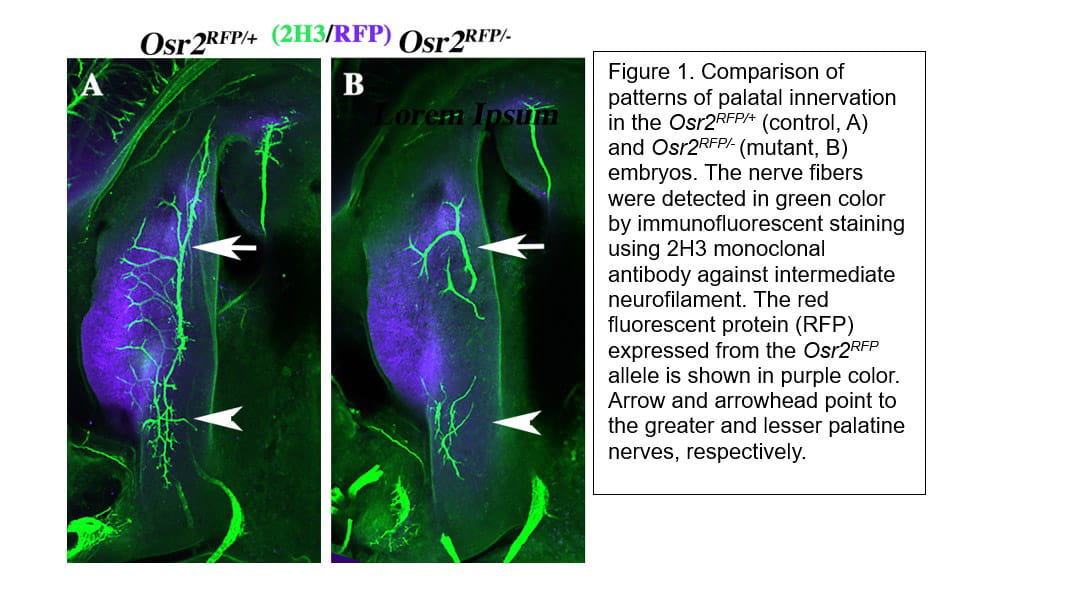
Cleft palate is one of the most common structural birth defects in humans. Most cleft palate patients in developed countries receive surgical repair and reconstruction of the palate, which restores many of the crucial functions of the palate but about 30% of these children still experience velopharyngeal dysfunction (VPD) and impairment of speech and/or hearing after treatment. Through mouse genetic studies, we identified the zinc finger transcription factor OSR2 as a key regulator of palate development and patterning. Combined analyses of genome-wide OSR2 binding, chromatin accessibility, and differential gene expression in the control and Osr2-/- embryos revealed that expression of several Sema3 family genes were aberrantly increased in the absence of OSR2. Immunofluorescent detection of nerve fibers revealed that Osr2 mutants exhibited disruption of palatal innervation, suggesting that OSR2 regulates palatal innervation through controlling the patterns of Sema3 gene expression. We have generated mice with disruptions in Sema3a, Sema3d, or both, using CRISPR/Cas9-mediated genome editing, and found that Sema3a and Sema3d play partly redundant roles in regulating craniofacial innervation.