Current Projects
Our Approach
We aim to study biology that is directly relevant to human health. For that reason, our research questions are based on findings identified in human patient samples and data. We then develop experimental model systems to investigate the underlying mechanisms and functional consequences of our findings in humans. The model systems we commonly use are the laboratory mouse and intestinal spheroid culture.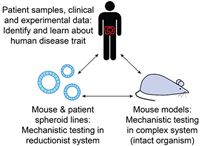
Research Topics
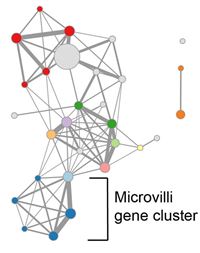
We recently identified epithelial defects associated with microvilli in non-inflamed Crohn’s disease intestinal tissue, including a down-regulated cluster of genes associated with the microvilli brush border and a histological decrease in microvilli length (VanDussen et al. Gastroenterol, 2018). These defects correlated with clinical parameters related to disease severity and response to treatment. We hypothesize that these microvilli defects contribute to chronic disease progression in a subset of Crohn’s patients. We are testing this hypothesis in expanded patient cohorts and in experimental model systems.
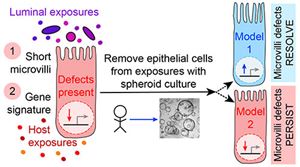
We do not yet know the biological driver of the microvilli-associated epithelial defects observed in Crohn’s disease intestinal tissue. We are taking a variety of approaches to determine if (and which) host or luminal exposures of the epithelial cell might be driving the altered expression of the microvilli signature genes and microvilli length. One approach is to determine if these microvilli-associated traits persist in patient-derived spheroid cell lines, in the absence of the host and luminal exposures. This knowledge will critically inform the design of future studies and potential therapeutic approaches to restore these epithelial cell functions.
Crohn's Disease
Defective function of intestinal epithelial cells occurs in chronic intestinal inflammatory diseases such as Crohn’s disease, which affects more than 750,000 Americans. Crohn’s disease symptoms include abdominal pain, rectal bleeding, diarrhea or sometimes constipation. The majority of Crohn’s disease patients will undergo one or more surgeries in their lifetime to remove damaged portions of their intestine because the current medications do not stop the long-term progression of the disease. Currently, the therapies for Crohn’s disease target inflammatory mediators and immune cell activation. There are no current therapies that act directly on intestinal epithelial cells. With our research, we aim to identify the defects in epithelial cell function that occur in Crohn’s disease intestine, to define the patient population subsets susceptible to these defects, and to investigate the underlying biological drivers and functional consequences of these defects. Our long-term goal is to develop personalized therapeutic approaches that restore epithelial function and promote intestinal healing in the subsets of Crohn’s patients with epithelial cell defects.
Learn more about Crohn’s disease and the goals of the inflammatory bowel disease research community here:

Long-term culture of intestinal epithelial cells
Long-term culture of intestinal tissue-derived epithelial cells became possible in 2009 following a report from the Hans Clevers laboratory that established the critical culture conditions (Sato et al. Nature, 2009). Major advantages of this technique are that non-transformed, non-tumorigenic epithelial cell lines can be established and studied. Since then, we (and others) have adapted the method by Sato et al. in a variety of ways to probe epithelial cell function.
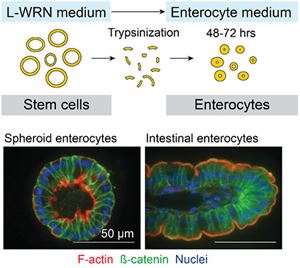
We use conditioned medium from the L-WRN cell line, engineered by the Stappenbeck laboratory (Miyoshi et al. Science, 2012; Miyoshi & Stappenbeck. Nat Protoc, 2013), to support the long-term growth of human and mouse tissue-derived epithelial stem cells as spheroids in a 3D matrix (Miyoshi & Stappenbeck. Nat Protoc, 2013; VanDussen et al. Gut, 2015). The L-WRN conditioned medium has reproducible stem cell-supporting activity from batch-to-batch (VanDussen et al. Stem Cell Res, 2019) and the resulting spheroids contain nearly all proliferating cells (Miyoshi et al. Science, 2012). Because of this, we are able to rapidly expand the number of intestinal epithelial cells to facilitate patient-based assays (VanDussen et al. Gut, 2015). Spheroid epithelial cell lines can be established from intestinal biopsy tissues collected during routine endoscopy procedures and stored long-term in biobanks from essentially any individual, including Crohn’s disease patients. These cell lines hold great promise for use in personalized medicine approaches.
With the L-WRN conditioned medium spheroid system, we can investigate epithelial cells grown on a variety of platforms and in a variety of cell states. Epithelial cells can be grown in a 2D monolayer on Transwell membranes to provide access to the apical membrane (Moon et al. Mucosal Immunol 2014; VanDussen et al. Gut, 2015); with 3D spheroids, the apical membrane faces the inner lumen and therefore is difficult to access. We can also induce the spheroid epithelial cells to differentiate to post-mitotic wound-associated epithelial cell and enterocyte lineages by altering the medium composition (Miyoshi & VanDussen et al. EMBO J, 2017). Importantly for us, the spheroid enterocytes develop a mature microvilli brush border in 48 hours.
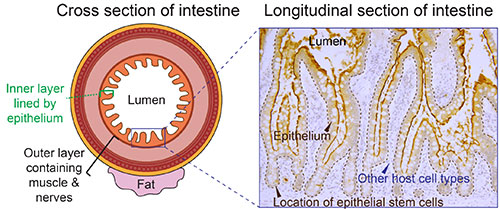
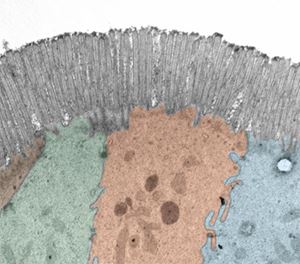
The Intestinal Epithelium Interface
The epithelium is a single layer of cells of approximately the thickness of a saran wrap sheet that lines the inner (luminal) side of the intestine. On one side of the epithelial interface, there are numerous host cell types, such as immune cells, muscle cells, and nerve cells. On the other side, there are dietary components, transient and resident microbes, and other external environmental factors. Intestinal epithelial cells promote human health through digestion and absorption of nutrients, by providing a barrier between the lumen and host compartments, and by sending and responding to signals from both of these compartments. Intestinal epithelial research explores the exciting interplay between host genetics, mucosal immunology, stem cell biology, and luminal environment influences such as diet and the microbiome!