Current Projects
Current Projects
Conditions like anaerobic exercise, sickle cell anemia, peripheral vascular disease and other cardiovascular disorders cause changes in protective sympathetic reflexes which are thought to be mediated by group III and IV muscle sensory neurons. However, these same fibers are also likely playing a role in ischemic muscle pain associated with these conditions. We do not currently have a comprehensive understanding of the sensitization that occurs in individual muscle afferent subtypes after ischemia or how changes in gene expression in the sensory ganglia may affect muscle afferent sensitization, which in turn may lead to the initiation or progression of pain states.
Project Goal
The goal of this project is to functionally characterize group III and IV muscle afferents in preclinical models of peripheral ischemia and determine the molecular mechanisms involved with changes in specific subpopulations of muscle afferents in males versus females. The information from these studies will provide a novel way to allow us to better understand the functional implications of changes in muscle sensory neurons and could be used to develop new and more appropriate therapeutic approaches for the management of altered cardiovascular reflexes and/or chronic musculoskeletal pain associated with ischemia.
Funding:
2024-2026 - "Sex dependent effects of prolactin receptor on muscle hypersensitivity following ischemic insult” - F31NS135995 NIH/NINDS (PI: Quijas)
2020-2025 - “Peripheral mechanisms of ischemic myalgia”- R01NS113965 NIH/NINDS
2020-2024 - "Electrical coupling of circulating immune cells to peripheral tissues”- R61/R33AR076808 NIH/NIAMS
2020 - “Mechanistic investigation of acute pain signal generation and chronification”- BHSAI/Henry Jackson Foundation
2018 - “Assessment of muscle pain after compound “X” injection in preclinical models”- Halozyme
2016 - “Linking sex differences in cardiovascular reflexes and pain perception” - NIH/NIAMS (PI: J. Ross)
2016 - “Role of GDNF in the dual modulation of nociception and cardiovascular reflexes during peripheral ischemia” - American Heart Association (PI: L. Queme)
2013 - 1R01AR064551 - NIH/NIAMS
2012 - International Association for the Study of Pain Early Career Grant
2012 - American Pain Society and the Rita Allen Foundation Award in Pain
 Neurochemical identity and response properties of an individually characterized chemosensitive muscle sensory neuron. |
|
 |
Ischemia causes skeletal muscle atrophy and tissue degradation. |
Surgery, preventative medicine and neonatal intensive care in addition to various pathological disorders in children can all lead to painful conditions making pediatric pain a significant clinical problem. Many of the pain therapies used in children are similar to that used in adults, yet it is well known that use of certain pharmacotherapies such as opiates or anti-inflammatories in children can have serious adverse effects.
Project Goal
The goal of this project is to comprehensively phenotype both cutaneous and muscle afferents during development and then determine the molecular mechanisms of sensitization that may occur after neonatal insults such as inflammation, incision or ischemia. These studies will not only allow us to obtain a significant amount of information on sensory neuron development, but will also inform us about the types of sensitization that occurs after neonatal injury and how this may alter sensory neuron responses later in life. This information may provide insight into the development of more appropriate pain therapies that target primary afferents in children.
Funding
2019-2029 - Sensitization of developing sensory neurons after incision”- NIH/NINDS
2013 -Trustee Scholar Award (Cincinnati Children's)
2013 -1R03HD077483 (NIH/NICHD)
2015 - Research, Innovation and Pilot Grant (Cincinnati Children's)
2017-2018 - Sensitization of developing sensory neurons during inflammation (NIH/NINDS)
 Neurochemical identity of a neonatal mechanoreceptor. |
Neurofibromatosis 1 (NF1) is an inherited tumor predisposition syndrome that affects ~1:3000 people. Peripheral nerve tumors in this disease can cause severe disfigurement and eventually compress vital structures, leading to serious or even fatal complications. In addition, pain is a major contributor to the decreased quality of life in patients with NF1. Since it is known that Schwann cells (SCs) and/or their precursors are the tumorigenic cells in NF1, it is likely that they also contribute to peripheral sensitization and pain. However, we do not know how SCs modulate pain under normal or pathological conditions.
Project Goal
The goal of this project is to determine the mechanisms by which alterations in SC function contribute to peripheral sensitization and pain during development and in genetically engineered mouse models of NF1. Results will allow us to determine how non-neuronal cells communicate with peripheral sensory neurons to modulate their functions and contribute to nociceptive processing.
Funding
2019 – “Modulation of nociception by Schwann cells in NF1”- Research, Innovation and Pilot Funding (Cincinnati Children's Hospital Medical Center)
2023-2025 - "Role of Schwann cells in NF1-related pain development"- Children's Tumor Foundation
2022-2025 - "The role of Schwann cells in the onset of pain in neurofibromatosis 1"- Children's Tumor Foundation (PI: Raut)
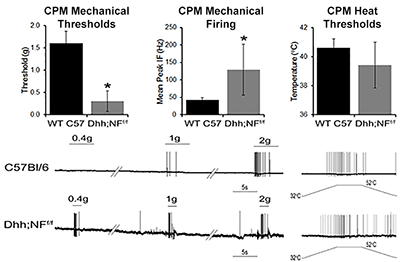 |
Sensory neuron (CPM) sensitization in a genetically engineered mouse model of neurofibromatosis 1 (Dhh;NFf/f). |



