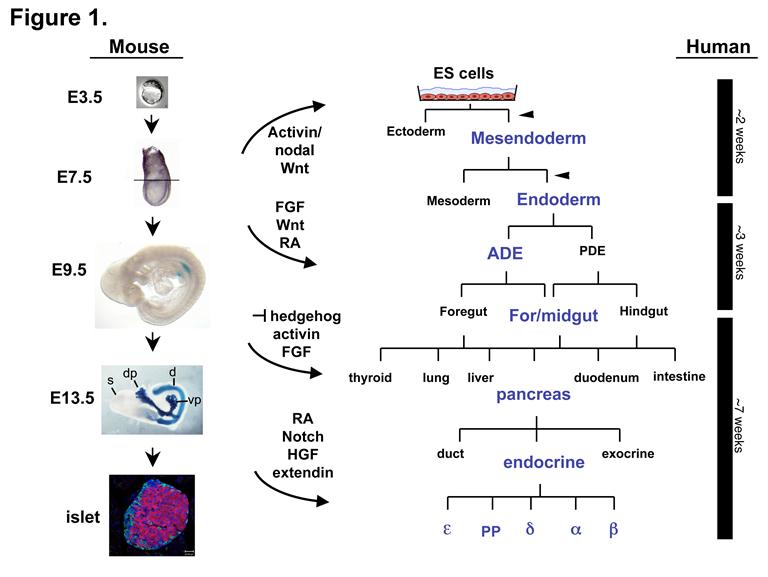
Differentiation of Pluripotent Embryonic Stem Cells into Endoderm Derivatives
We are using the signals and genes that direct endoderm and pancreas differentiation in the embryo to direct the differentiation of pluripotent embryonic stem (ES) cells into therapeutically important endoderm derivatives, such as insulin-producing beta cells. The goal of this work is to generate cells that could be used in a transplantation-based therapy to treat type 1 diabetes (figure 1 from Spence and Wells, 2007). Embryonic stem cells have enormous therapeutic potential because they are genetically unprogrammed and can form all cell types of the body.
Differentiation of Human PSCs into Intestinal Tissue
Studies in embryonic development have guided successful efforts to direct the differentiation of human embryonic and induced pluripotent stem cells (PSCs) into specific organ cell types in vitro. For example, human PSCs have been differentiated into monolayer cultures of liver hepatocytes and pancreatic endocrine cells, which have therapeutic efficacy in animal models of liver disease and diabetes, respectively. However, the generation of complex three-dimensional organ tissues in vitro remains a major challenge for translational studies.
We have established a robust and efficient process to direct the differentiation of human PSCs into intestinal tissue in vitro using a temporal series of growth factor manipulations to mimic embryonic intestinal development. This involved activin-induced definitive endoderm (DE) formation, FGF / Wnt-induced posterior endoderm pattering, hindgut specification and morphogenesis, and a pro-intestinal culture system to promote intestinal growth, morphogenesis and cytodifferentiation.
The resulting three-dimensional intestinal “organoids” consisted of a polarized, columnar epithelium that was patterned into villus-like structures and crypt-like proliferative zones that expressed intestinal stem cell markers. The epithelium contained functional enterocytes, as well as goblet, Paneth and enteroendocrine cells.
Using this culture system as a model to study human intestinal development, we identified that the combined activity of Wnt3a and FGF4 is required for hindgut specification whereas FGF4 alone is sufficient to promote hindgut morphogenesis. Our data suggest that human intestinal stem cells form de novo during development.
Lastly we determined that NEUROG3, a pro-endocrine transcription factor that is mutated in enteric anendocrinosis, is both necessary and sufficient for human enteroendocrine cell development in vitro.
In conclusion, PSC-derived human intestinal tissue should allow for unprecedented studies of human intestinal development and disease.
View publications from the Wells Lab in PubMed.